Richardson, K. et al. Earth beyond six of nine planetary boundaries. Sci. Adv. 9, eadh2458 (2023).
Google Scholar
IPCC Climate Change 2023: Synthesis Report (eds Core Writing Team, Lee, H. & Romero, J.) https://www.ipcc.ch/report/ar6/syr/downloads/report/IPCC_AR6_SYR_LongerReport.pdf (IPCC, 2023).
Romanello, M. et al. The 2023 report of the Lancet Countdown on health and climate change: the imperative for a health-centred response in a world facing irreversible harms. Lancet 402, 2346–2394 (2023).
Google Scholar
Romanello, M. et al. The 2021 report of the Lancet Countdown on health and climate change: code red for a healthy future. Lancet 398, 1619–1662 (2021).
Google Scholar
World Health Organization. Ambient (outdoor) air pollution. World Health Organization https://www.who.int/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health (2024).
Anderson, J. O., Thundiyil, J. G. & Stolbach, A. Clearing the air: a review of the effects of particulate matter air pollution on human health. J. Med. Toxicol. 8, 166–175 (2012).
Google Scholar
Lenzen, M. et al. The environmental footprint of health care: a global assessment. Lancet Planet. Health 4, e271–e279 (2020).
Google Scholar
Grand View Research. Medical plastics market size, share a trends analysis report by application (medical device packaging, medical components, orthopedic implant packaging), and segment forecasts. 2019–2025. Grand View Research https://www.grandviewresearch.com/industry-analysis/medical-plastics-market (2019).
Landrigan, P. J. et al. The Minderoo–Monaco Commission on plastics and human health. Ann. Glob. Health 89, 23 (2023).
Google Scholar
Brighton and Sussex Medical School, Centre for Sustainable Healthcare, and UK Health Alliance on Climate Change. Green Surgery: Reducing the Environmental Impact of Surgical Care https://ukhealthalliance.org/sustainable-healthcare/green-surgery-report/ (2023).
White, S. M. et al. Principles of environmentally-sustainable anaesthesia: a global consensus statement from the World Federation of Societies of Anaesthesiologists. Anaesthesia 77, 201–212 (2022).
Google Scholar
Wilkinson, A. & Woodcock, A. The environmental impact of inhalers for asthma: a green challenge and a golden opportunity. Br. J. Clin. Pharmacol. 88, 3016–3022 (2022).
Google Scholar
Chung, J. W. & Meltzer, D. O. Estimate of the carbon footprint of the US health care sector. JAMA 302, 1970–1972 (2009).
Google Scholar
Tennison, I. et al. Health care’s response to climate change: a carbon footprint assessment of the NHS in England. Lancet Planet. Health 5, e84–e92 (2021).
Google Scholar
Wu, R. The carbon footprint of the Chinese health-care system: an environmentally extended input–output and structural path analysis study. Lancet Planet. Health 3, e413–e419 (2019).
Google Scholar
Malik, A., Lenzen, M., McAlister, S. & McGain, F. The carbon footprint of Australian health care. Lancet Planet. Health 2, e27–e35 (2018).
Google Scholar
Nansai, K., Fry, J., Malik, A., Takayanagi, W. & Kondo, N. Carbon footprint of Japanese health care services from 2011 to 2015. Resour. Conserv. Recycling 152, 104525 (2020).
Marsh, K., Ganz, M., Nørtoft, E., Lund, N. & Graff-Zivin, J. Incorporating environmental outcomes into a health economic model. Int. J. Technol. Assess. Health Care 32, 400–406 (2016).
Google Scholar
Fordham, R. et al. Effective diabetes complication management is a step toward a carbon-efficient planet: an economic modeling study. BMJ Open. Diabetes Res. Care 8, e001017 (2020).
Google Scholar
Bhutta, M. F. Our over-reliance on single-use equipment in the operating theatre is misguided, irrational and harming our planet. Ann. R. Coll. Surg. Engl. 103, 709–712 (2021).
Google Scholar
MacNeill, A. J. et al. Transforming the medical device industry: road map to a circular economy. Health Aff. 39, 2088–2097 (2020).
Sridharan, S., Kumar, M., Singh, L., Bolan, N. S. & Saha, M. Microplastics as an emerging source of particulate air pollution: a critical review. J. Hazard. Mater. 418, 126245 (2021).
Google Scholar
Karapetrova, A. et al. Exploring microplastic distribution in Western North American snow. J. Hazard. Mater. 480, 136126 (2024).
Google Scholar
Vivekanand, A. C., Mohapatra, S. & Tyagi, V. K. Microplastics in aquatic environment: challenges and perspectives. Chemosphere 282, 131151 (2021).
Google Scholar
Surendran, U., Jayakumar, M., Raja, P., Gopinath, G. & Chellam, P. V. Microplastics in terrestrial ecosystem: sources and migration in soil environment. Chemosphere 318, 137946 (2023).
Google Scholar
Pabortsava, K. & Lampitt, R. S. High concentrations of plastic hidden beneath the surface of the Atlantic Ocean. Nat. Commun. 11, 4073 (2020).
Google Scholar
Massoud, M. A., Abdallah, C., Merhbi, F., Khoury, R. & Ghanem, R. Development and application of a prioritization and rehabilitation decision support tool for uncontrolled waste disposal sites in developing countries. Integr. Environ. Assess. Manag. 19, 436–445 (2023).
Google Scholar
Chisholm, J. M. et al. Sustainable waste management of medical waste in African developing countries: a narrative review. Waste Manag. Res. 39, 1149–1163 (2021).
Google Scholar
Adeniran, A. A., Ayesu-Koranteng, E. & Shakantu, W. A review of the literature on the environmental and health impact of plastic waste pollutants in sub-Saharan Africa. Pollutants 2, 531–545 (2022).
Martuzzi, M., Mitis, F. & Forastiere, F. Inequalities, inequities, environmental justice in waste management and health. Eur. J. Public. Health 20, 21–26 (2010).
Google Scholar
La Merrill, M. A. et al. Consensus on the key characteristics of endocrine-disrupting chemicals as a basis for hazard identification. Nat. Rev. Endocrinol. 16, 45–57 (2020).
Google Scholar
Gore, A. C. et al. EDC-2: the endocrine society’s second scientific statement on endocrine-disrupting chemicals. Endocr. Rev. 36, E1–e150 (2015).
Google Scholar
Padmanabhan, V., Sarma, H. N., Savabieasfahani, M., Steckler, T. L. & Veiga-Lopez, A. Developmental reprogramming of reproductive and metabolic dysfunction in sheep: native steroids vs. environmental steroid receptor modulators. Int. J. Androl. 33, 394–404 (2010).
Google Scholar
Woodruff, T. J. Health effects of fossil fuel-derived endocrine disruptors. N. Engl. J. Med. 390, 922–933 (2024).
Google Scholar
Ghassabian, A. & Trasande, L. Disruption in thyroid signaling pathway: a mechanism for the effect of endocrine-disrupting chemicals on child neurodevelopment. Front. Endocrinol. 9, 204 (2018).
Heindel, J. J. et al. NIEHS/FDA CLARITY-BPA research program update. Reprod. Toxicol. 58, 33–44 (2015).
Google Scholar
Hao, C., Cheng, X., Xia, H. & Ma, X. The endocrine disruptor mono-(2-ethylhexyl) phthalate promotes adipocyte differentiation and induces obesity in mice. Biosci. Rep. 32, 619–629 (2012).
Google Scholar
Ziv-Gal, A., Wang, W., Zhou, C. & Flaws, J. A. The effects of in utero bisphenol A exposure on reproductive capacity in several generations of mice. Toxicol. Appl. Pharmacol. 284, 354–362 (2015).
Google Scholar
Harding, C., Van Loon, J., Moons, I., De Win, G. & Du Bois, E. Design opportunities to reduce waste in operating rooms. Sustainability 13, 2207 (2021).
Zimmermann, L. et al. Plastic products leach chemicals that induce in vitro toxicity under realistic use conditions. Environ. Sci. Technol. 55, 11814–11823 (2021).
Google Scholar
Chang, W. H., Herianto, S., Lee, C. C., Hung, H. & Chen, H. L. The effects of phthalate ester exposure on human health: a review. Sci. Total Environ. 786, 147371 (2021).
Google Scholar
Ma, Y. et al. The adverse health effects of bisphenol A and related toxicity mechanisms. Environ. Res. 176, 108575 (2019).
Google Scholar
Trasande, L. et al. Estimating burden and disease costs of exposure to endocrine-disrupting chemicals in the European union. J. Clin. Endocrinol. Metab. 100, 1245–1255 (2015).
Google Scholar
Jacobson, M. H., Woodward, M., Bao, W., Liu, B. & Trasande, L. Urinary bisphenols and obesity prevalence among US children and adolescents. J. Endocr. Soc. 3, 1715–1726 (2019).
Google Scholar
Mallozzi, M., Leone, C., Manurita, F., Bellati, F. & Caserta, D. Endocrine disrupting chemicals and endometrial cancer: an overview of recent laboratory evidence and epidemiological studies. Int. J. Environ. Res. Public. Health 14, 334 (2017).
Google Scholar
Environment and Climate Change Canada, Health Canada. Screening assessment — phthalate substance grouping. Environment and Climate Change Canada https://www.canada.ca/en/environment-climate-change/services/evaluating-existing-substances/screening-assessment-phthalate-substance-grouping.html (2020).
Rowdhwal, S. S. S. & Chen, J. Toxic effects of di-2-ethylhexyl phthalate: an overview. Biomed. Res. Int. 2018, 1750368 (2018).
Google Scholar
Koch, H. M., Preuss, R. & Angerer, J. Di(2-ethylhexyl)phthalate (DEHP): human metabolism and internal exposure — an update and latest results. Int. J. Androl. 29, 155–165 (2006).
Google Scholar
Šimunović, A., Tomić, S. & Kranjčec, K. Medical devices as a source of phthalate exposure: a review of current knowledge and alternative solutions. Arh. Hig. Rada Toksikol. 73, 179–190 (2022).
Google Scholar
Fong, J. P., Lee, F. J., Lu, I. S., Uang, S. N. & Lee, C. C. Estimating the contribution of inhalation exposure to di-2-ethylhexyl phthalate (DEHP) for PVC production workers, using personal air sampling and urinary metabolite monitoring. Int. J. Hyg. Environ. Health 217, 102–109 (2014).
Google Scholar
Al-Saleh, I. et al. The cumulative risk assessment of phthalates exposure in preterm neonates. Int. J. Hyg. Environ. Health 248, 114112 (2023).
Google Scholar
European Commission: Directorate-General for Health and Food Safety. Opinion on the safety of medical devices containing DEHP-plasticized PVC or other plasticizers on neonates and other groups possibly at risk (2015 update). European Commission https://op.europa.eu/en/publication-detail/-/publication/1bee9152-0253-11e7-8a35-01aa75ed71a1/language-en (2016).
Hauser, R. et al. Urinary phthalate metabolite concentrations and reproductive outcomes among women undergoing in vitro fertilization: results from the EARTH study. Environ. Health Perspect. 124, 831–839 (2016).
Google Scholar
Al-Saleh, I. et al. The relationships between urinary phthalate metabolites, reproductive hormones and semen parameters in men attending in vitro fertilization clinic. Sci. Total Environ. 658, 982–995 (2019).
Google Scholar
Zhang, X., Flaws, J. A., Spinella, M. J. & Irudayaraj, J. The relationship between typical environmental endocrine disruptors and kidney disease. Toxics 11, 32 (2022).
Google Scholar
Martinez-Arguelles, D. B. & Papadopoulos, V. Mechanisms mediating environmental chemical-induced endocrine disruption in the adrenal gland. Front. Endocrinol. 6, 29 (2015).
Zhang, Y., Lyu, L., Tao, Y., Ju, H. & Chen, J. Health risks of phthalates: a review of immunotoxicity. Environ. Pollut. 313, 120173 (2022).
Google Scholar
Liu, Y. et al. An insight into sex-specific neurotoxicity and molecular mechanisms of DEHP: a critical review. Environ. Pollut. 316, 120673 (2023).
Google Scholar
Beg, M. A. & Sheikh, I. A. Endocrine disruption: structural interactions of androgen receptor against di(2-ethylhexyl) phthalate and its metabolites. Toxics 8, 115 (2020).
Google Scholar
Rouiller-Fabre, V. et al. Nuclear receptors and endocrine disruptors in fetal and neonatal testes: a gapped landscape. Front. Endocrinol. 6, 58 (2015).
Wang, W., Craig, Z. R., Basavarajappa, M. S., Gupta, R. K. & Flaws, J. A. Di(2-ethylhexyl) phthalate inhibits growth of mouse ovarian antral follicles through an oxidative stress pathway. Toxicol. Appl. Pharmacol. 258, 288–295 (2012).
Google Scholar
Corton, J. C. & Lapinskas, P. J. Peroxisome proliferator-activated receptors: mediators of phthalate ester-induced effects in the male reproductive tract? Toxicol. Sci. 83, 4–17 (2005).
Google Scholar
Ren, H. et al. Characterization of peroxisome proliferator-activated receptor α — independent effects of PPARα activators in the rodent liver: di-(2-ethylhexyl) phthalate also activates the constitutive-activated receptor. Toxicol. Sci. 113, 45–59 (2010).
Google Scholar
Jiang, J., Ma, L., Yuan, L., Wang, X. & Zhang, W. Study on developmental abnormalities in hypospadiac male rats induced by maternal exposure to di-n-butyl phthalate (DBP). Toxicology 232, 286–293 (2007).
Google Scholar
Martinez-Arguelles, D. B. & Papadopoulos, V. Prenatal phthalate exposure: epigenetic changes leading to lifelong impact on steroid formation. Andrology 4, 573–584 (2016).
Google Scholar
Geens, T., Goeyens, L. & Covaci, A. Are potential sources for human exposure to bisphenol-A overlooked? Int. J. Hyg. Environ. Health 214, 339–347 (2011).
Google Scholar
Bernier, M. R. & Vandenberg, L. N. Handling of thermal paper: implications for dermal exposure to bisphenol A and its alternatives. PLoS ONE 12, e0178449 (2017).
Google Scholar
Löfroth, M., Ghasemimehr, M., Falk, A. & von Steyern, P. V. Bisphenol A in dental materials — existence, leakage and biological effects. Heliyon 5, e01711 (2019).
Google Scholar
Health Canada. Bisphenol A (BPA) in Canadians. Health Canada https://www.canada.ca/content/dam/hc-sc/documents/services/environmental-workplace-health/reports-publications/environmental-contaminants/human-biomonitoring-resources/bisphenol-a-canadians/bpa-eng.pdf (2021).
ECHA (European Chemicals Agency. Bisphenols. ECHA https://echa.europa.eu/hot-topics/bisphenols (2021).
US Food and Drug Administration. Bisphenol A (BPA): use in food contact application. FDA.gov https://www.fda.gov/food/food-packaging-other-substances-come-contact-food-information-consumers/bisphenol-bpa-use-food-contact-application (2014).
Lawson, C. et al. Gene expression in the fetal mouse ovary is altered by exposure to low doses of bisphenol A. Biol. Reprod. 84, 79–86 (2011).
Google Scholar
Alonso-Magdalena, P. et al. Bisphenol-A acts as a potent estrogen via non-classical estrogen triggered pathways. Mol. Cell. Endocrinol. 355, 201–207 (2012).
Google Scholar
Hafezi, S. A. & Abdel-Rahman, W. M. The endocrine disruptor bisphenol A (BPA) exerts a wide range of effects in carcinogenesis and response to therapy. Curr. Mol. Pharmacol. 12, 230–238 (2019).
Google Scholar
Wang, H. et al. Anti-androgenic mechanisms of bisphenol A involve androgen receptor signaling pathway. Toxicology 387, 10–16 (2017).
Google Scholar
MacKay, H. & Abizaid, A. A plurality of molecular targets: the receptor ecosystem for bisphenol-A (BPA). Hormones Behav. 101, 59–67 (2018).
Google Scholar
Fan, X., Katuri, G. P., Caza, A. A., Rasmussen, P. E. & Kubwabo, C. Simultaneous measurement of 16 bisphenol A analogues in house dust and evaluation of two sampling techniques. Emerg. Contam. 7, 1–9 (2021).
Google Scholar
Zheng, J., Tian, L. & Bayen, S. Chemical contaminants in canned food and can-packaged food: a review. Crit. Rev. Food Sci. Nutr. 63, 2687–2718 (2023).
Google Scholar
Pelch, K. et al. A scoping review of the health and toxicological activity of bisphenol A (BPA) structural analogues and functional alternatives. Toxicology 424, 152235 (2019).
Google Scholar
Rajkumar, A. et al. Elucidation of the effects of bisphenol A and structural analogs on germ and steroidogenic cells using single cell high-content imaging. Toxicol. Sci. 180, 224–238 (2021).
Google Scholar
Barra, N. G. et al. Increased gut serotonin production in response to bisphenol A structural analogs may contribute to their obesogenic effects. Am. J. Physiol. Endocrinol. Metab. 323, E80–E091 (2022).
Google Scholar
Glüge, J. et al. An overview of the uses of per- and polyfluoroalkyl substances (PFAS). Environ. Sci. Process. Impacts 22, 2345–2373 (2020).
Google Scholar
EFSA Panel on Contaminants in the Food Chain (EFSA CONTAM Panel) Risk to human health related to the presence of perfluoroalkyl substances in food. EFSA J. 18, e06223 (2020).
Ding, N., Harlow, S. D., Randolph, J. F. Jr, Loch-Caruso, R. & Park, S. K. Perfluoroalkyl and polyfluoroalkyl substances (PFAS) and their effects on the ovary. Hum. Reprod. Update 26, 724–752 (2020).
Google Scholar
National Academies of Sciences, Engineering, and Medicine. Guidance on PFAS exposure, testing, and clinical follow-up. The National Academies Press https://www.nationalacademies.org/our-work/guidance-on-pfas-testing-and-health-outcomes (2022).
Buck Louis, G. M. et al. Endocrine disruptors and neonatal anthropometry, NICHD fetal growth studies — singletons. Environ. Int. 119, 515–526 (2018).
Google Scholar
Boyd, R. I. et al. Toward a mechanistic understanding of poly-and perfluoroalkylated substances and cancer. Cancers 14, 2919 (2022).
Google Scholar
Das, K. P. et al. Perfluoroalkyl acids-induced liver steatosis: effects on genes controlling lipid homeostasis. Toxicology 378, 37–52 (2017).
Google Scholar
Dixit, G. & Prabhu, A. The pleiotropic peroxisome proliferator activated receptors: regulation and therapeutics. Exp. Mol. Pathol. 124, 104723 (2022).
Google Scholar
Zhao, B. et al. Exposure to perfluorooctane sulfonate in utero reduces testosterone production in rat fetal Leydig cells. PLoS ONE 9, e78888 (2014).
Google Scholar
Sonkar, R., Kay, M. K. & Choudhury, M. PFOS modulates interactive epigenetic regulation in first-trimester human trophoblast cell line HTR-8/SVneo. Chem. Res. Toxicol. 32, 2016–2027 (2019).
Google Scholar
Rashid, F., Ramakrishnan, A., Fields, C. & Irudayaraj, J. Acute PFOA exposure promotes epigenomic alterations in mouse kidney tissues. Toxicol. Rep. 7, 125–132 (2020).
Google Scholar
Blum, A. et al. The Madrid statement on poly-and perfluoroalkyl substances (PFASs). Environ. Health Perspect. 123, A107–A111 (2015).
Google Scholar
Underwriters’ Laboratories. UL 94 Tests for flammability of plastic materials for parts in devices and appliances. Underwriters’ Laboratories https://www.shopulstandards.com/ProductDetail.aspx?productId=UL94 (2007).
Van der Veen, I. & de Boer, J. Phosphorus flame retardants: properties, production, environmental occurrence, toxicity and analysis. Chemosphere 88, 1119–1153 (2012).
Google Scholar
Hoffman, K., Garantziotis, S., Birnbaum, L. S. & Stapleton, H. M. Monitoring indoor exposure to organophosphate flame retardants: hand wipes and house dust. Environ. Health Perspect. 123, 160–165 (2015).
Google Scholar
Wang, X., Hales, B. F. & Robaire, B. Effects of flame retardants on ovarian function. Reprod. Toxicol. 102, 10–23 (2021).
Google Scholar
Harley, K. G. et al. PBDE concentrations in women’s serum and fecundability. Environ. Health Perspect. 118, 699–704 (2010).
Google Scholar
Lefèvre, P. L. et al. Exposure of female rats to an environmentally relevant mixture of brominated flame retardants targets the ovary, affecting folliculogenesis and steroidogenesis. Biol. Reprod. 94, 1–11 (2016).
Allais, A. et al. In utero and lactational exposure to flame retardants disrupts rat ovarian follicular development and advances puberty. Toxicol. Sci. 175, 197–209 (2020).
Google Scholar
Dingemans, M. M., van den Berg, M. & Westerink, R. H. Neurotoxicity of brominated flame retardants:(in) direct effects of parent and hydroxylated polybrominated diphenyl ethers on the (developing) nervous system. Environ. Health Perspect. 119, 900–907 (2011).
Google Scholar
Hales, B. F. & Robaire, B. Effects of brominated and organophosphate ester flame retardants on male reproduction. Andrology 8, 915–923 (2020).
Google Scholar
Blum, A. et al. Organophosphate ester flame retardants: are they a regrettable substitution for polybrominated diphenyl ethers? Environ. Sci. Technol. Lett. 6, 638–649 (2019).
Google Scholar
Percy, Z. et al. Concentrations and loadings of organophosphate and replacement brominated flame retardants in house dust from the home study during the PBDE phase-out. Chemosphere 239, 124701 (2020).
Google Scholar
Peng, Y. et al. Review on typical organophosphate diesters (di-OPEs) requiring priority attention: formation, occurrence, toxicological, and epidemiological studies. J. Hazard. Mater. 460, 132426 (2023).
Google Scholar
Wang, X. et al. The effects of organophosphate esters used as flame retardants and plasticizers on granulosa, Leydig, and spermatogonial cells analyzed using high-content imaging. Toxicol. Sci. 186, 269–287 (2022).
Google Scholar
Li, Z., Robaire, B. & Hales, B. F. The organophosphate esters used as flame retardants and plasticizers affect H295R adrenal cell phenotypes and functions. Endocrinology 164, bqad119 (2023).
Google Scholar
Wang, X., Lee, E., Hales, B. F. & Robaire, B. Organophosphate esters disrupt steroidogenesis in kgn human ovarian granulosa cells. Endocrinology 164, bqad089 (2023).
Google Scholar
Hartmann, N. B. et al. Are we speaking the same language? Recommendations for a definition and categorization framework for plastic debris. Environ. Sci. Technol. 53, 1039–1047 (2019).
Google Scholar
Anagnosti, L., Varvaresou, A., Pavlou, P., Protopapa, E. & Carayanni, V. Worldwide actions against plastic pollution from microbeads and microplastics in cosmetics focusing on European policies. Has the issue been handled effectively? Mar. Pollut. Bull. 162, 111883 (2021).
Google Scholar
Cole, M., Lindeque, P., Halsband, C. & Galloway, T. S. Microplastics as contaminants in the marine environment: a review. Mar. Pollut. Bull. 62, 2588–2597 (2011).
Google Scholar
Amec Foster Wheeler Environment and Infrastructure UK Limited. Intentionally added microplastics in products; final report. European Commission https://www.r10labs.com/wp-content/uploads/39168-Intentionally-added-microplastics-Final-report-20171020.pdf (2017).
Wiesinger, H., Wang, Z. & Hellweg, S. Deep dive into plastic monomers, additives, and processing aids. Environ. Sci. Technol. 55, 9339–9351 (2021).
Google Scholar
Field, D. T. et al. Microplastics in the surgical environment. Environ. Int. 170, 107630 (2022).
Google Scholar
Jenner, L. C. et al. Detection of microplastics in human lung tissue using μFTIR spectroscopy. Sci. Total Environ. 831, 154907 (2022).
Google Scholar
Ippoliti, F. et al. Comparative spallation performance of silicone versus Tygon extracorporeal circulation tubing. Interact. Cardiovasc. Thorac. Surg. 29, 685–692 (2019).
Google Scholar
Barron, D. et al. Particle spallation induced by blood pumps in hemodialysis tubing sets. Artif. Organs 10, 226–235 (1986).
Google Scholar
Bommer, J., Ritz, E. & Waldherr, R. Silicone-induced splenomegaly: treatment of pancytopenia by splenectomy in a patient on hemodialysis. N. Engl. J. Med. 305, 1077–1079 (1981).
Google Scholar
Teo, A. J. T. et al. Polymeric biomaterials for medical implants and devices. ACS Biomater. Sci. Eng. 2, 454–472 (2016).
Google Scholar
Tarafdar, A., Xie, J., Gowen, A., O’Higgins, A. C. & Xu, J. L. Advanced optical photothermal infrared spectroscopy for comprehensive characterization of microplastics from intravenous fluid delivery systems. Sci. Total Environ. 929, 172648 (2024).
Google Scholar
Passos, R. S. et al. Microplastics and nanoplastics in haemodialysis waters: emerging threats to be in our radar. Environ. Toxicol. Pharmacol. 102, 104253 (2023).
Google Scholar
Shastri, P. V. Toxicology of polymers for implant contraceptives for women. Contraception 65, 9–13 (2002).
Google Scholar
Pothupitiya, J. U., Zheng, C. & Saltzman, W. M. Synthetic biodegradable polyesters for implantable controlled-release devices. Expert. Opin. Drug Deliv. 19, 1351–1364 (2022).
Google Scholar
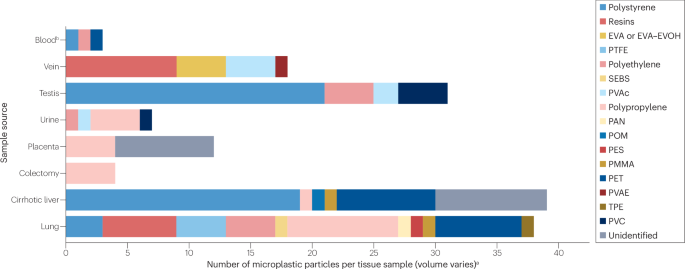
Schwabl, P. et al. Detection of various microplastics in human stool: a prospective case series. Ann. Intern. Med. 171, 453–457 (2019).
Google Scholar
Amato-Lourenço, L. F. et al. Presence of airborne microplastics in human lung tissue. J. Hazard. Mater. 416, 126124 (2021).
Google Scholar
Leslie, H. A. et al. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 163, 107199 (2022).
Google Scholar
Ibrahim, Y. S. et al. Detection of microplastics in human colectomy specimens. JGH Open 5, 116–121 (2021).
Google Scholar
Horvatits, T. et al. Microplastics detected in cirrhotic liver tissue. eBioMedicine 82, 104147 (2022).
Google Scholar
Ragusa, A. et al. Plasticenta: first evidence of microplastics in human placenta. Environ. Int. 146, 106274 (2021).
Google Scholar
Ragusa, A. et al. Raman microspectroscopy detection and characterisation of microplastics in human breastmilk. Polymers 14, 2700 (2022).
Google Scholar
Yang, Y. et al. Detection of various microplastics in patients undergoing cardiac surgery. Environ. Sci. Technol. 57, 10911–10918 (2023).
Google Scholar
Rotchell, J. M. et al. Detection of microplastics in human saphenous vein tissue using μFTIR: a pilot study. PLoS ONE 18, e0280594 (2023).
Google Scholar
Zhao, W., van der Voet, E., Huppes, G. & Zhang, Y. Comparative life cycle assessments of incineration and non-incineration treatments for medical waste. Int. J. Life Cycle Assess. 14, 114–121 (2009).
Google Scholar
Pironti, C. et al. First evidence of microplastics in human urine, a preliminary study of intake in the human body. Toxics 11, 40 (2022).
Google Scholar
Li, Z. et al. Identification and analysis of microplastics in human lower limb joints. J. Hazard. Mater. 461, 132640 (2024).
Google Scholar
Danopoulos, E., Twiddy, M., West, R. & Rotchell, J. M. A rapid review and meta-regression analyses of the toxicological impacts of microplastic exposure in human cells. J. Hazard. Mater. 427, 127861 (2022).
Google Scholar
Zolotova, N., Kosyreva, A., Dzhalilova, D., Fokichev, N. & Makarova, O. Harmful effects of the microplastic pollution on animal health: a literature review. PeerJ 10, e13503 (2022).
Google Scholar
Dewan, P. A., Stefanek, W. & Byard, R. W. Long-term histological response to intravenous Teflon and silicone in a rat model. Pediatric Surg. Int. 10, 129–133 (1995).
Luo, T. et al. Maternal polystyrene microplastic exposure during gestation and lactation altered metabolic homeostasis in the dams and their F1 and F2 offspring. Environ. Sci. Technol. 53, 10978–10992 (2019).
Google Scholar
Hou, B., Wang, F., Liu, T. & Wang, Z. Reproductive toxicity of polystyrene microplastics: in vivo experimental study on testicular toxicity in mice. J. Hazard. Mater. 405, 124028 (2021).
Google Scholar
Jin, H. et al. Polystyrene microplastics induced male reproductive toxicity in mice. J. Hazard. Mater. 401, 123430 (2021).
Google Scholar
An, R. et al. Polystyrene microplastics cause granulosa cells apoptosis and fibrosis in ovary through oxidative stress in rats. Toxicology 449, 152665 (2021).
Google Scholar
Liu, Z. et al. Polystyrene microplastics induced female reproductive toxicity in mice. J. Hazard. Mater. 424, 127629 (2022).
Google Scholar
Deng, Y. et al. Microplastics release phthalate esters and cause aggravated adverse effects in the mouse gut. Environ. Int. 143, 105916 (2020).
Google Scholar
Eisenakh, I. A., Bondarev, O. I., Mozes, V. G., Lapii, G. A. & Lushnikova, E. L. Features of in vitro degradation and physical properties of a biopolymer and in vivo tissue reactions in comparison with polypropylene. Bull. Exp. Biol. Med. 170, 88–92 (2020).
Google Scholar
Spiwak, A. J., Horbal, A., Leatherbury, R. & Hansford, D. J. Extracorporeal tubing in the roller pump raceway: physical changes and particulate generation. J. Extra Corpor. Technol. 40, 188–192 (2008).
Google Scholar
Orenstein, J. M., Sato, N., Aaron, B., Buchholz, B. & Bloom, S. Microemboli observed in deaths following cardiopulmonary bypass surgery: silicone antifoam agents and polyvinyl chloride tubing as sources of emboli. Hum. Pathol. 13, 1082–1090 (1982).
Google Scholar
Brewer, A., Dror, I. & Berkowitz, B. The mobility of plastic nanoparticles in aqueous and soil environments: a critical review. ACS Est. Water 1, 48–57 (2021).
Google Scholar
Torres-Ruiz, M. et al. Neurotoxicity and endocrine disruption caused by polystyrene nanoparticles in zebrafish embryo. Sci. Total Environ. 874, 162406 (2023).
Google Scholar
Amereh, F. et al. Thyroid endocrine status and biochemical stress responses in adult male Wistar rats chronically exposed to pristine polystyrene nanoplastics. Toxicol. Res. 8, 953–963 (2019).
Google Scholar
Valdez-Carrillo, M., Abrell, L., Ramírez-Hernández, J., Reyes-López, J. A. & Carreón-Diazconti, C. Pharmaceuticals as emerging contaminants in the aquatic environment of Latin America: a review. Environ. Sci. Pollut. Res. Int. 27, 44863–44891 (2020).
Google Scholar
Wilkinson, J. L. et al. Pharmaceutical pollution of the world’s rivers. Proc. Natl Acad. Sci. USA 119, e2113947119 (2022).
Google Scholar
Niemi, L. et al. Assessing hospital impact on pharmaceutical levels in a rural ‘source-to-sink’ water system. Sci. Total Environ. 737, 139618 (2020).
Google Scholar
Aydın, S., Ulvi, A., Bedük, F. & Aydın, M. E. Pharmaceutical residues in digested sewage sludge: occurrence, seasonal variation and risk assessment for soil. Sci. Total Environ. 817, 152864 (2022).
Google Scholar
Daouk, S. et al. Prioritization methodology for the monitoring of active pharmaceutical ingredients in hospital effluents. J. Environ. Manag. 160, 324–332 (2015).
Google Scholar
Ort, C., Lawrence, M. G., Rieckermann, J. & Joss, A. Sampling for pharmaceuticals and personal care products (ppcps) and illicit drugs in wastewater systems: are your conclusions valid? A critical review. Environ. Sci. Technol. 44, 6024–6035 (2010).
Google Scholar
Langford, K. H. & Thomas, K. V. Determination of pharmaceutical compounds in hospital effluents and their contribution to wastewater treatment works. Environ. Int. 35, 766–770 (2009).
Google Scholar
Hernández-Tenorio, R., González-Juárez, E., Guzmán-Mar, J. L., Hinojosa-Reyes, L. & Hernández-Ramírez, A. Review of occurrence of pharmaceuticals worldwide for estimating concentration ranges in aquatic environments at the end of the last decade. J. Hazard. Mater. Adv. 8, 100172 (2022).
Spilsbury, F., Kisielius, V., Bester, K. & Backhaus, T. Ecotoxicological mixture risk assessment of 35 pharmaceuticals in wastewater effluents following post-treatment with ozone and/or granulated activated carbon. Sci. Total Environ. 906, 167440 (2024).
Google Scholar
De Falco, M. & Laforgia, V. Combined effects of different endocrine-disrupting chemicals (edcs) on prostate gland. Int. J. Environ. Res. Public Health 18, 9772 (2021).
Google Scholar
Sachs, H. C. The transfer of drugs and therapeutics into human breast milk: an update on selected topics. Pediatrics 132, e796–e809 (2013).
Google Scholar
Newbold, R. R., Padilla-Banks, E. & Jefferson, W. N. Adverse effects of the model environmental estrogen diethylstilbestrol are transmitted to subsequent generations. Endocrinology 147, S11–S17 (2006).
Google Scholar
Fu, Q., Sanganyado, E., Ye, Q. & Gan, J. Meta-analysis of biosolid effects on persistence of triclosan and triclocarban in soil. Environ. Pollut. 210, 137–144 (2016).
Google Scholar
Wang, M. et al. Multi-class determination of steroid hormones and antibiotics in fatty hotpot ingredients by pressurized liquid extraction and liquid chromatography-tandem mass spectrometry. J. Pharm. Biomed. Anal. 171, 193–203 (2019).
Google Scholar
Álvarez-Muñoz, D. et al. Occurrence of pharmaceuticals and endocrine disrupting compounds in macroalgaes, bivalves, and fish from coastal areas in Europe. Environ. Res. 143, 56–64 (2015).
Google Scholar
Godfray, H. C. J. et al. A restatement of the natural science evidence base on the effects of endocrine disrupting chemicals on wildlife. Proc. Biol. Sci. 286, 20182416 (2019).
Google Scholar
Ebele, A. J., Abou-Elwafa Abdallah, M. & Harrad, S. Pharmaceuticals and personal care products (PPCPs) in the freshwater aquatic environment. Emerg. Contam. 3, 1–16 (2017).
Kasprzyk-Hordern, B., Dinsdale, R. M. & Guwy, A. J. The occurrence of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs in surface water in South Wales, UK. Water Res. 42, 3498–3518 (2008).
Google Scholar
Adelakun, S. A., Ukwenya, V. O. & Akintunde, O. W. Vitamin B12 ameliorate tramadol-induced oxidative stress, endocrine imbalance, apoptosis and NO/iNOS/NF-κB expression in Sprague Dawley rats through regulatory mechanism in the pituitary–gonadal axis. Tissue Cell 74, 101697 (2022).
Google Scholar
Sehonova, P. et al. Effects of antidepressants with different modes of action on early life stages of fish and amphibians. Environ. Pollut. 254, 112999 (2019).
Google Scholar
Munro, K. et al. Evaluation of combined sewer overflow impacts on short-term pharmaceutical and illicit drug occurrence in a heavily urbanised tidal river catchment (London, UK). Sci. Total Environ. 657, 1099–1111 (2019).
Google Scholar
Paulis, M. G., Hafez, E. M. & El-Tahawy, N. F. Toxicity and postwithdrawal effects of ketamine on the reproductive function of male albino rats: hormonal, histological, and immunohistochemical study. Hum. Exp. Toxicol. 39, 1054–1065 (2020).
Google Scholar
Reichert, J. F., Souza, D. M. & Martins, A. F. Antipsychotic drugs in hospital wastewater and a preliminary risk assessment. Ecotoxicol. Environ. Saf. 170, 559–567 (2019).
Google Scholar
Frédéric, O. & Yves, P. Pharmaceuticals in hospital wastewater: their ecotoxicity and contribution to the environmental hazard of the effluent. Chemosphere 115, 31–39 (2014).
Google Scholar
Ilgin, S. The adverse effects of psychotropic drugs as an endocrine disrupting chemicals on the hypothalamic-pituitary regulation in male. Life Sci. 253, 117704 (2020).
Google Scholar
Elizalde-Velázquez, G. A. & Gómez-Oliván, L. M. Occurrence, toxic effects and removal of metformin in the aquatic environments in the world: recent trends and perspectives. Sci. Total Environ. 702, 134924 (2020).
Google Scholar
Tavlo, M. et al. Hypothesis: metformin is a potential reproductive toxicant. Front. Endocrinol. 13, 1000872 (2022).
United Nations. The United Nations World Water Development Report 2021: Valuing Water. UNESCO https://unesdoc.unesco.org/ark:/48223/pf0000375724 (2021).
European Commission Communication and Information Resource Centre for Administrations. Drinking water parameter cooperation project. European Commission https://circabc.europa.eu/d/a/workspace/SpacesStore/7d664fea-50ed-4f6b-8eaf-8179900de47b/WHO%20Parameter%20Report.pdf (2017).
Karnjanapiboonwong, A. et al. Occurrence of PPCPs at a wastewater treatment plant and in soil and groundwater at a land application site. Water Air Soil Pollut. 216, 257–273 (2011).
Google Scholar
Weatherly, L. M. & Gosse, J. A. Triclosan exposure, transformation, and human health effects. J. Toxicol. Environ. Health B 20, 447–469 (2017).
Google Scholar
Rochester, J. R., Bolden, A. L., Pelch, K. E. & Kwiatkowski, C. F. Potential developmental and reproductive impacts of triclocarban: a scoping review. J. Toxicol. 2017, 9679738 (2017).
Google Scholar
Geer, L. A. et al. Association of birth outcomes with fetal exposure to parabens, triclosan and triclocarban in an immigrant population in Brooklyn, New York. J. Hazard. Mater. 323, 177–183 (2017).
Google Scholar
Wei, L. et al. Triclosan/triclocarban levels in maternal and umbilical blood samples and their association with fetal malformation. Clin. Chim. Acta 466, 133–137 (2017).
Google Scholar
Parveen, N., Chowdhury, S. & Goel, S. Environmental impacts of the widespread use of chlorine-based disinfectants during the COVID-19 pandemic. Environ. Sci. Pollut. Res. Int. 29, 85742–85760 (2022).
Google Scholar
Pérez-Albaladejo, E. et al. Genotoxicity and endocrine disruption potential of haloacetic acids in human placental and lung cells. Sci. Total Environ. 879, 162981 (2023).
Google Scholar
MacNeill, A. J., McGain, F. & Sherman, J. D. Planetary health care: a framework for sustainable health systems. Lancet Planet. Health 5, e66–e68 (2021).
Google Scholar
Galaviz, K. I., Narayan, K. M. V., Lobelo, F. & Weber, M. B. Lifestyle and the prevention of type 2 diabetes: a status report. Am. J. Lifestyle Med. 12, 4–20 (2018).
Google Scholar
Sharma, R., Biedenharn, K. R., Fedor, J. M. & Agarwal, A. Lifestyle factors and reproductive health: taking control of your fertility. Reprod. Biol. Endocrinol. 11, 66 (2013).
Google Scholar
Rogers, N. T. et al. Changes in soft drinks purchased by British households associated with the UK soft drinks industry levy: a controlled interrupted time series analysis. BMJ Open 13, e077059 (2023).
Google Scholar
Haw, J. S. et al. Long-term sustainability of diabetes prevention approaches: a systematic review and meta-analysis of randomized clinical trials. JAMA Intern. Med. 177, 1808–1817 (2017).
Google Scholar
Black, L. J., Seamans, K. M., Cashman, K. D. & Kiely, M. An updated systematic review and meta-analysis of the efficacy of vitamin D food fortification. J. Nutr. 142, 1102–1108 (2012).
Google Scholar
Kruger, M. C. et al. The effect of a fortified milk drink on vitamin D status and bone turnover in post-menopausal women from South East Asia. Bone 46, 759–767 (2010).
Google Scholar
The Endocrine Society. Advocacy and policy. The Endocrine Society https://www.endocrine.org/advocacy (2022).
Di Renzo, G. C. et al. International Federation of Gynecology and Obstetrics opinion on reproductive health impacts of exposure to toxic environmental chemicals. Int. J. Gynaecol. Obstet. 131, 219–225 (2015).
Google Scholar
Appelberg, K. et al. Cost-effectiveness of newborn screening for phenylketonuria and congenital hypothyroidism. J. Pediatr. 256, 38–43 (2023).
Google Scholar
Haque, M. M. et al. Cost-effectiveness of diagnosis and treatment of early gestational diabetes mellitus: economic evaluation of the TOBOGM study, an international multicenter randomized controlled trial. eClinicalMedicine 71, 102610 (2024).
Google Scholar
Liu, D., Shi, Q., Liu, C., Sun, Q. & Zeng, X. Effects of endocrine-disrupting heavy metals on human health. Toxics 11, 322 (2023).
Google Scholar
Sasaki, T. et al. Hospital-based screening to detect patients with cadmium nephropathy in cadmium-polluted areas in Japan. Environ. Health Prev. Med. 24, 8 (2019).
Google Scholar
Horiguchi, H. et al. Latest status of cadmium accumulation and its effects on kidneys, bone, and erythropoiesis in inhabitants of the formerly cadmium-polluted Jinzu River Basin in Toyama, Japan, after restoration of rice paddies. Int. Arch. Occup. Environ. Health 83, 953–970 (2010).
Google Scholar
Lagerweij, G. R. et al. Impact of preventive screening and lifestyle interventions in women with a history of preeclampsia: a micro-simulation study. Eur. J. Prev. Cardiol. 27, 1389–1399 (2020).
Google Scholar
Mortimer, F., Isherwood, J., Wilkinson, A. & Vaux, E. Sustainability in quality improvement: redefining value. Future Healthc. J. 5, 88–93 (2018).
Google Scholar
Samudera, W. S., Efendi, F. & Indarwati, R. Effect of community and peer support based healthy lifestyle program (CP-HELP) on self care behavior and fasting blood glucose in patient with type 2 diabetes mellitus. J. Diabetes Metab. Disord. 20, 193–199 (2021).
Google Scholar
Thomas, D., Elliott, E. J. & Naughton, G. A. Exercise for type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2006, CD002968 (2006).
Google Scholar
Rakhra, V., Galappaththy, S. L., Bulchandani, S. & Cabandugama, P. K. Obesity and the Western diet: how we got here. Mo. Med. 117, 536–538 (2020).
Google Scholar
Pradhan, P., Reusser, D. E. & Kropp, J. P. Embodied greenhouse gas emissions in diets. PLoS ONE 8, e62228 (2013).
Google Scholar
Gryka, A., Broom, J. & Rolland, C. Global warming: is weight loss a solution? Int. J. Obes. 36, 474–476 (2012).
Google Scholar
Willett, W. et al. Food in the anthropocene: the EAT–Lancet Commission on healthy diets from sustainable food systems. Lancet 393, 447–492 (2019).
Google Scholar
Lin, X., Wang, S. & Huang, J. The association between the EAT–Lancet diet and diabetes: a systematic review. Nutrients 15, 4462 (2023).
Google Scholar
Ehrenkranz, J. Point-of-care endocrine diagnostics. Endocrinol. Metab. Clin. North. Am. 46, 615–630 (2017).
Google Scholar
Bell, C. et al. Clinic for multimorbidity: an innovative approach to integrate general practice and specialized health care services. Int. J. Integr. Care 23, 25 (2023).
Google Scholar
Ongaro, A. E. et al. Engineering a sustainable future for point-of-care diagnostics and single-use microfluidic devices. Lab Chip 22, 3122–3137 (2022).
Google Scholar
Goodwin, V. A. et al. Implementing a patient-initiated review system for people with rheumatoid arthritis: a prospective, comparative service evaluation. J. Eval. Clin. Pract. 22, 439–445 (2016).
Google Scholar
Kershaw, V. F., Chainrai, M. & Radley, S. C. Patient initiated follow up in obstetrics and gynaecology: a systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 272, 123–129 (2022).
Google Scholar
Turley, M. et al. Use of electronic health records can improve the health care industry’s environmental footprint. Health Aff. 30, 938–946 (2011).
Purohit, A., Smith, J. & Hibble, A. Does telemedicine reduce the carbon footprint of healthcare? A systematic review. Future Healthc. J. 8, e85–e91 (2021).
Google Scholar
Wang, E. Y. et al. Environmental emissions reduction of a preoperative evaluation center utilizing telehealth screening and standardized preoperative testing guidelines. Resour. Conserv. Recycling 171, 105652 (2021).
Google Scholar
Grealey, J. et al. The carbon footprint of bioinformatics. Mol. Biol. Evol. 39, msac034 (2022).
Google Scholar
Dunne, H., Jones, A. & Okorie, M. Combatting climate change using education and training in pharmacology and therapeutics. Br. J. Clin. Pharmacol. 89, 1518–1520 (2023).
Google Scholar
Klonoff, D. C. et al. The diabetes technology society green diabetes initiative. J. Diabetes Sci. Technol. 14, 507–512 (2020).
Google Scholar
Keil, M., Viere, T., Helms, K. & Rogowski, W. The impact of switching from single-use to reusable healthcare products: a transparency checklist and systematic review of life-cycle assessments. Eur. J. Public. Health 33, 56–63 (2023).
Google Scholar
Sanchez, S. A., Eckelman, M. J. & Sherman, J. D. Environmental and economic comparison of reusable and disposable blood pressure cuffs in multiple clinical settings. Resour. Conserv. Recycling 155, 104643 (2020).
Rizan, C., Bhutta, M. F., Reed, M. & Lillywhite, R. The carbon footprint of waste streams in a UK hospital. J. Clean. Prod. 286, 125446 (2021).
Google Scholar
Lopes, D. G., Duarte, I. A., Antunes, M. & Fonseca, V. F. Effects of antidepressants in the reproduction of aquatic organisms: a meta-analysis. Aquat. Toxicol. 227, 105569 (2020).
Google Scholar
Petry, S. F., Petry, F. W., Petry, J. K., Gäth, S. & Heinemann, L. Diabetes technology and waste: a real-world study in a specialized practice in Germany. J. Diabetes Sci. Technol. https://doi.org/10.1177/19322968241257004 (2024).
Google Scholar
Poudel, R. S. et al. Assessment of insulin injection practice among diabetes patients in a tertiary healthcare centre in Nepal: a preliminary study. J. Diabetes Res. 2017, 8648316 (2017).
Google Scholar
Ishtiaq, O. et al. Disposal of syringes, needles, and lancets used by diabetic patients in Pakistan. J. Infect. Public Health 5, 182–188 (2012).
Google Scholar
Atukorala, K. R., Wickramasinghe, S. I., Sumanasekera, R. D. N. & Wickramasinghe, K. H. Practices related to sharps disposal among diabetic patients in Sri Lanka. Asia Pac. Fam. Med. 17, 12 (2018).
Google Scholar
Furth, R., Audrey, A. & Gumti, K. Safer insulin needle use and disposal. Int. J. Infect. Control. https://doi.org/10.3396/ijic.v6i2.5037 (2009).
Google Scholar
Anastas, P. T. & Zimmerman, J. B. Design through the 12 principles of green engineering. Environ. Sci. Technol. 37, 94A–101A (2003).
Google Scholar
Fiksel, J. Design for Environment: a Guide to Sustainable Product Development 2nd edn (McGraw-Hill Education, 2009).
De Soete, W., Jimenez-Gonzalez, C., Dahlin, P. & Dewulf, J. Challenges and recommendations for environmental sustainability assessments of pharmaceutical products in the healthcare sector. Green. Chem. 90, 41 (2017).
Milanesi, M., Runfola, A. & Guercini, S. Pharmaceutical industry riding the wave of sustainability: review and opportunities for future research. J. Clean. Prod. 261, 121204 (2020).
Rodríguez-Serin, H. et al. Literature review: evaluation of drug removal techniques in municipal and hospital wastewater. Int. J. Environ. Res. Public. Health 19, 13105 (2022).
Google Scholar
Mauch, J., Kronsbein, A. L., Putschew, A., Lewandowski, J. & Hilt, S. Periphyton in urban freshwater facilitates transformation of trace organic compounds: a case study on iodinated contrast media. Front. Environ. Sci. 11, 1142591 (2023).
Martinez-Vaz, B. M. et al. Wastewater bacteria remediating the pharmaceutical metformin: genomes, plasmids and products. Front. Bioeng. Biotechnol. 10, 1086261 (2022).
Google Scholar
Nguyen, K. T. et al. Green diabetes summit 2021. J. Diabetes Sci. Technol. 16, 233–247 (2022).
Google Scholar
World Health Organization. Alliance for transformative action on climate and health (ATACH). World Health Organization https://www.who.int/initiatives/alliance-for-transformative-action-on-climate-and-health/country-commitments (2022).
Arasaradnam, R. Our new campaign: sustainability and climate change. Royal College of Physicians https://www.rcp.ac.uk/news-and-media/news-and-opinion/our-new-campaign-sustainability-and-climate-change (2023).
Planetary Health Report Card. 2022–2023 Summary Report; an international health student initiative. Planetary Health Report Card https://phreportcard.org/wp-content/uploads/2023/04/PHRC-2023-Med-Summary-Report-FINAL.pdf (2023).
Sherman, J. D. et al. Net zero healthcare: a call for clinician action. Br. Med. J. 374, n1323 (2021).
Gordon, D. & Zuegge, K. L. Greenwashing in health care marketing. ASA Monit. 84, 18–21 (2020).
Li, D. et al. Anticancer drugs in the aquatic ecosystem: environmental occurrence, ecotoxicological effect and risk assessment. Environ. Int. 153, 106543 (2021).
Google Scholar
Palmer, B. D. & Palmer, S. K. Vitellogenin induction by xenobiotic estrogens in the red-eared turtle and African clawed frog. Environ. Health Perspect. 103, 19–25 (1995).
Google Scholar
Williams, T. D. et al. Evaluation of the reproductive effects of tamoxifen citrate in partial and full life-cycle studies using fathead minnows (Pimephales promelas). Environ. Toxicol. Chem. 26, 695–707 (2007).
Google Scholar
Sun, L., Zha, J., Spear, P. A. & Wang, Z. Tamoxifen effects on the early life stages and reproduction of Japanese medaka (Oryzias latipes). Environ. Toxicol. Pharmacol. 24, 23–29 (2007).
Google Scholar
Pinto, C. A., Fonseca, B. M. & Sá, S. I. Effects of chronic tamoxifen treatment in female rat sexual behaviour. Heliyon 8, e12362 (2022).
Google Scholar
Diaz-Camal, N., Cardoso-Vera, J. D., Islas-Flores, H., Gómez-Oliván, L. M. & Mejía-García, A. Consumption and ocurrence of antidepressants (SSRIs) in pre- and post-COVID-19 pandemic, their environmental impact and innovative removal methods: a review. Sci. Total Environ. 829, 154656 (2022).
Google Scholar
Ford, A. T. & Fong, P. P. The effects of antidepressants appear to be rapid and at environmentally relevant concentrations. Environ. Toxicol. Chem. 35, 794–798 (2016).
Google Scholar
Weinberger, J. II & Klaper, R. Environmental concentrations of the selective serotonin reuptake inhibitor fluoxetine impact specific behaviors involved in reproduction, feeding and predator avoidance in the fish Pimephales promelas (fathead minnow). Aquat. Toxicol. 151, 77–83 (2014).
Google Scholar
Whitlock, S. E., Pereira, M. G., Shore, R. F., Lane, J. & Arnold, K. E. Environmentally relevant exposure to an antidepressant alters courtship behaviours in a songbird. Chemosphere 211, 17–24 (2018).
Google Scholar
Pawluski, J. L. Perinatal selective serotonin reuptake inhibitor exposure: impact on brain development and neural plasticity. Neuroendocrinology 95, 39–46 (2012).
Google Scholar
Sengar, A. & Vijayanandan, A. Comprehensive review on iodinated X-ray contrast media: complete fate, occurrence, and formation of disinfection byproducts. Sci. Total Environ. 769, 144846 (2021).
Google Scholar
Nowak, A., Pacek, G. & Mrozik, A. Transformation and ecotoxicological effects of iodinated X-ray contrast media. Rev. Environ. Sci. Biotechnol. 19, 337–354 (2020).
Andreucci, M., Solomon, R. & Tasanarong, A. Side effects of radiographic contrast media: pathogenesis, risk factors, and prevention. Biomed. Res. Int. 2014, 741018 (2014).
Google Scholar
Manso, J. et al. Safety and efficacy of prophylactic treatment for hyperthyroidism induced by iodinated contrast media in a high-risk population. Front. Endocrinol. 14, 1154251 (2023).
Koagouw, W., Arifin, Z., Olivier, G. W. J. & Ciocan, C. High concentrations of paracetamol in effluent dominated waters of Jakarta Bay, Indonesia. Mar. Pollut. Bull. 169, 112558 (2021).
Google Scholar
Xia, L., Zheng, L. & Zhou, J. L. Effects of ibuprofen, diclofenac and paracetamol on hatch and motor behavior in developing zebrafish (Danio rerio). Chemosphere 182, 416–425 (2017).
Google Scholar
Ciślak, M., Kruszelnicka, I., Zembrzuska, J. & Ginter-Kramarczyk, D. Estrogen pollution of the European aquatic environment: a critical review. Water Res. 229, 119413 (2023).
Google Scholar
Arlos, M. J. et al. Modeling the exposure of wild fish to endocrine active chemicals: potential linkages of total estrogenicity to field-observed intersex. Water Res. 139, 187–197 (2018).
Google Scholar
Mackuľak, T. et al. Hospital wastewaters treatment: fenton reaction vs. BDDE vs. ferrate(VI). Environ. Sci. Pollut. Res. 26, 31812–31821 (2019).
Bodík, M. et al. Searching for the correlations between the use of different groups of pharmaceuticals from wastewaters. Ecotoxicol. Environ. Saf. 228, 112973 (2021).
Google Scholar
Zhao, Q. et al. Detection and characterization of microplastics in the human testis and semen. Sci. Total Environ. 877, 162713 (2023).
Google Scholar