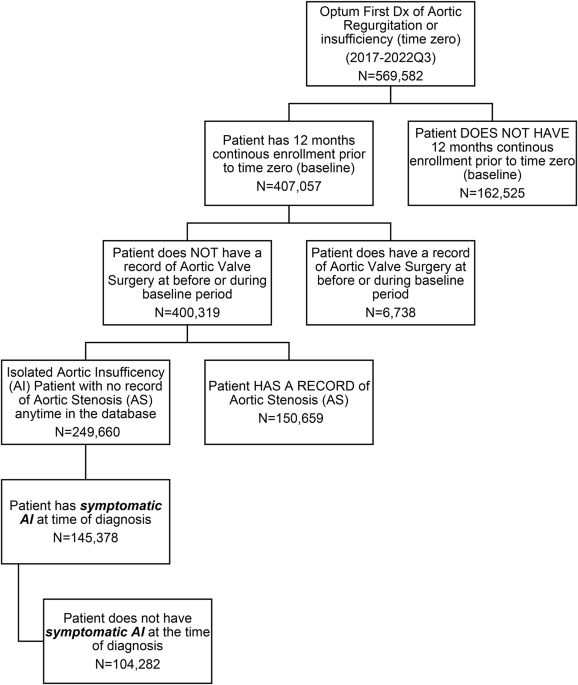
In this retrospective, isolated aortic valve failure, 58.23% (145,378) had symptomatic disease at the time of diagnosis (zero time). Of the remaining 41.86% (104,282) of patients, almost half (47.34%) were asymptomatic at the time of AI diagnosis, within five years. This rapid progression is consistent with recent evidence that adverse left ventricular remodeling may occur at lower thresholds than previously recognized, with the terminal reduction volume index of 45 mL/ m2>45 ml/m2 and excretion rate <60% may identify early dysfunction. This study highlights the substantial healthcare burden generally associated with AI, particularly for those with symptomatic AI. Patients with symptomatic AI at the time of diagnosis were older and sicker at baseline than those with asymptomatic AI. After adjusting for differences between baseline demographics and chronic comorbidities, we found that the care burden in the symptomatic cohort was consistently higher than that in the asymptomatic cohort on the following measures: mortality rate (27) %), Home Health Care Utilization (64% vs. 47%), SNF Admission (16% vs. 7%), IP Visit (0.49 vs. 0.40), CV IP Visit (0.15 vs. 0.10), ER Visit (0.62 vs. 0.46), Hospital days (3.8 vs. 3.0) and OP visits (6.5 vs. 6.2). Overall, there was a total of statistically significant differences (total adjusted annual costs (SAI $54,982 vs. $48,588), highlighting the cost burden arising from the disease to society. Home health care utilization rate (64% vs. 47%) and SNF hospitalizations in the symptomatic cohort (16% vs. 7%) highlight downstream functional declines associated with these outcomes: delay or withholding.
In particular, only a small percentage of patients (AAI 4.18% and SAI 0.58%) will undergo aortic valve replacement, regardless of whether AI patients are symptomatic beyond the five-year period of this study. Particularly for patients with symptoms, receiving AVR according to current clinical guidelines has been severely and underutilized by the cardiovascular community17. Given that AI presents a heavy healthcare burden and timely interventions with AVR are excellent treatments, understanding the causes of such low surgical intervention rates in both study cohorts is a crucial factor. , is important to address this huge clinical gap.
Our findings showed that timely intervention with SAVR could reduce overall medical use and cost burden for patients with symptomatic aortic reflux. According to the analysis, patients who received SAVR within 12 months of SAR diagnosis provided significant mortality benefits compared to those who did not. Additionally, those who underwent surgery within 5 years of diagnosis were associated with a lower mortality rate (SAR without SAVR was 15% versus SAR with SAR and 39% with P <.0001 (The mortality rate reached). The analysis also showed low healthcare access and costs for 12 months among SAR patients with or without SAR ($41,399 without SAVR)
There are many barriers to surgery that may explain such a low invasion of AVR interventions in this patient population. First, an increased co-existence burden in the symptomatic AI group could lead physicians/providers to consider the causes of symptoms in patients secondary to comorbidities rather than AI. Second, there may be resistance to referring patients with symptomatic AI to a surgeon. This resistance to surgical referral may be driven in part by the patient himself. Therefore, it may seem desirable to have medical therapy that is more continuous and escalating than surgery, for both the physician/provider and the patient. Our symptomatic cohort had a higher burden of comorbidities (ECI 5.83 vs. 3.28) and cardiovascular complications, but these differences alone do not fully explain the significantly lower AVR rates. yeah. A recent analysis of 4,608 symptomatic AI patients found that 25.7% had SAVR within one year of diagnosis. This suggests that the majority of symptomatic patients can undergo surgery safely than what we observed in our cohort (<1%). Further research is needed to understand whether specific comorbidities combinations or other clinical factors facilitate surgical decision-making in these patients. Recent advances in imaging have improved the ability to detect early myocardial dysfunction7, but this has not been translated into previous interventions. This is particularly concerning, as recent studies indicate that patients meeting class I surgical signs may already have progressive progression that limits the benefits of the intervention. 12.
Third, the lack of transcatheter valve options to treat AI – like aortic stenosis that can be treated with surgical AVR or transcatheter AVR, can contribute to delays in surgical referral. Interestingly, from a technical point of view, surgical AVRs of AI are less complicated than AVRs of aortic stenosis. This increases the likelihood of increased calcification of all elements in the aortic root complex, including the aortic valve and coronary arteries and arteries. Valsalva's sinuses also tend to be smaller. Therefore, from a surgical AVR perspective, symptomatic AI patients may have surgical therapy options that may not be technically complicated than aortic stenosis surgery. A better understanding of surgical outcomes in patients with symptomatic AI will help us understand the relative survival benefits of timely interventions.
In a recent analysis of 4,608 symptomatic AI patients, 25.7% received surgical AVR within one year. Of those 25.7%, the mortality rate was 9% per year, with 24% remaining untreated at 17. Another study investigated guidelines-based surgical outcomes for symptomatic AI and found that Class I indications for surgical interventions had a higher long-term risk than class IIA or class IIB labelling. This suggests that patients meet the class I indications according to current guidelines, given the high mortality rates seen in medical management even in asymptomatic severe AI, and surgical AVR There may be too much progress in the disease process to maximize profits. This supports the premise that previous interventions in this patient population may improve survival benefits. Collectively, these studies highlight the importance of early recognition of asymptomatic and symptomatic AI in patients and the importance of timely referrals for surgical therapy considerations. Furthermore, identifying hurdles leading to such a large clinical gap in patient care is important to improve care for isolated AI patients.
Limitations of this study include the fact that AI was identified through a source of automated data that rely on coding. This may be biased in terms of overcode or undercoding. For example, there is no severity of the AR through echocardiographic data. Therefore, our study relied on diagnostic codes and health claims data. This limitation may include patients with less severe disease in our cohort, contributing to the observed reduction in SAVR rates. Known confounding factors (generally only controls between the magnitudes of common diseases, and ideally lack the ability to control unknown confounding factors through mechanisms such as randomization, stratification, and matching. Statistical modeling was used to control for the potential confounding effects of known variables with differences between groups. The statistical model controls several factors, but also includes management databases such as echo contrast results. Several variables not included in the model were not controlled. However, the strength of this study was that the data showed the characteristics and outcomes of real-world patients across the country compared to evidence from controlled clinical trials. It's something that reflects.