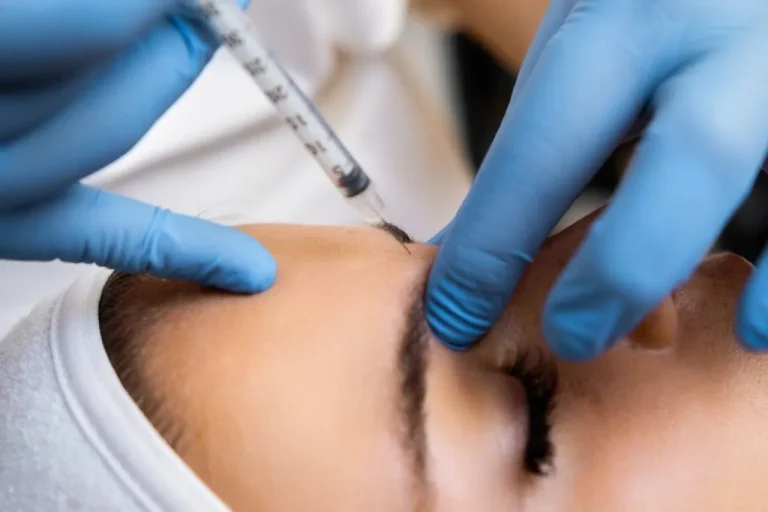
Nurse prescribers must consult with people face-to-face before issuing non-surgical cosmetic medicines, under new guidance being brought in by the Nursing and Midwifery Council (NMC).
The regulator this week announced that it was updating its position on the remote prescribing of medicines such as anti-wrinkle and weight-loss injections, after research found that in-person consultations would better protect the public.
“Our updated position on the remote prescribing of non-surgical cosmetic medicines is in the best interests of public safety and protection”
Anne Trotter
From 1 June 2025, nursing and midwifery prescribers will be required to consult with people face-to-face before issuing prescriptions.
They must undertake and document an appropriate clinical assessment of the intended recipient of the medicine before they prescribe any products.
It follows concerns that previous guidance allowed nurses to prescribe these medicines without any face-to-face contact with the recipient and without adequate access to their medical records.
Once prescribed by regulated professionals, the medicines can be administered by another professional who does not have a prescribing qualification such as a beautician.
Among the products being prescribed in this way are certain anti-wrinkle and weight-loss drugs.
The NMC said its new guidance would “better align the NMC with other health and care regulators” like the General Medical Council, which requires its registrants to carry out face-to-face consultations before issuing these drugs.
As such, the document has set out that it is “not appropriate” to prescribe non-surgical cosmetic medicines by remote methods, including telephone, email, online or video.
This is applicable to any initial consultation carried out by a nurse prescriber, as well as any subsequent consultations and follow-ups.
It follows research that was carried out by the NMC to better understand people’s perception of remote prescribing and the impact any potential changes would have on those administering and receiving non-surgical cosmetic medicines.
In 2024, independent research agency Thinks carried out research on behalf of the regular, in which it spoke to members of the public who had undergone these procedures and those who had not.
In addition, the NMC held a roundtable event with regulated healthcare professionals who prescribe non-surgical cosmetic medicines; regulated healthcare professionals who administer them; unregulated practitioners who administer them, such as beauticians; and cosmetic business owners.
The research found that, overall, people supported the NMC strengthening its position to better protect the public and agreed it would improve safety for people using services.
It revealed that many people who underwent non-surgical cosmetic procedures had not fully considered how the medicine was obtained when the person administering or injecting it was not a healthcare professional.
Meanwhile, the research found that people perceived procedures to be overly accessible, taking place in unregulated environments and with uncertainty about whether those administering or injecting medicines had sufficient training.
Some participants, especially business owners, thought that video consultations should be permitted and that a requirement for face-to-face consultations felt outdated.
Anne Trotter, NMC assistant director of education and standards, said: “Following our research and engagement, we’re confident that our updated position on the remote prescribing of non-surgical cosmetic medicines is in the best interests of public safety and protection.
“Nursing and midwifery prescribers provide competent and effective care to people every day.
“Face-to-face consultations will further improve their ability to assess people holistically and ensure non-surgical cosmetic medicines are prescribed as safely and appropriately.
“We’ll continue to engage with our stakeholders, professionals, employers and the public as we bring this new requirement into effect.”
The move to update the NMC guidance comes against a backdrop of tightening regulations around cosmetic procedures, which have been rising in popularity in the UK.
More on cosmetic procedures and nurse prescribing